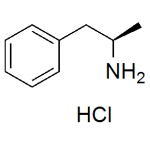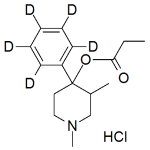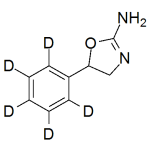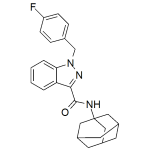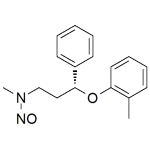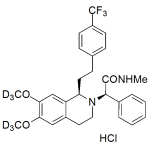Chemtos
Service, Quality, Speed
Chemtos
Service, Quality, Speed
> A
L-Amphetamine Hydrochloride (Levoamphetamine HCl)
High purity L-Amphetamine Hydrochloride (Levoamphetamine HCl) includes detailed Certificate of Analysis and copies of supporting analytical data.
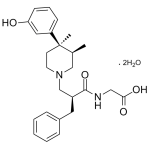
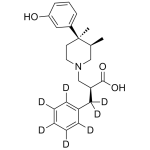
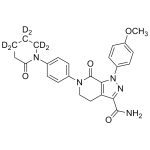
Alvimopan
High purity Alvimopan Dihydrate includes a comprehensive Certificate of Analysis and all supporting analytical data
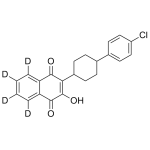
Atovaquone Labeled d4 (mix of cis and trans)
High purity Atovaquone Labeled d4 includes a comprehensive Certificate of Analysis and all supporting analytical data
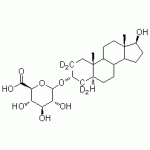
5α-Androstane-3α,17ß-diol-3-O-glucuronide-d4
High purity 5α-Androstane-3α,17ß-diol-3-O-glucuronide labeled d4 (Dihydroandrosterone-3-O-glucuronide labeled d4, 3α-Diol-G labeled d4, (3α,5α,17β)-17-Hydroxyandrostan-3-yl β-D-glucopyranuronate labeled d4, (3α,5α,17β)-17-Hydroxyandrostan-3-yl β-D-glucopyranuronate labeled d4) includes a comprehensive Certificate of Analysis and all supporting analytical data
Alvimopan Metabolite Labeled d7
High purity Alvimopan Metabolite Labeled d7 includes a comprehensive Certificate of Analysis and all supporting analytical data
Alphaprodine-d5 HCl
High purity Alphaprodine labeled d5 Hydrochloride includes a comprehensive Certificate of Analysis and all supporting analytical data (Chiral HPLC or %ee value not included in CoA)
Aminorex -d5
High purity Aminorex labeled d5 includes a comprehensive Certificate of Analysis and all supporting analytical data
FUB-APINACA
High purity FUB-APINACA, FUB-AKB48, AFUBINACA, AFB-48 includes a comprehensive Certificate of Analysis and all supporting analytical data
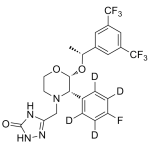
Aprepitant labeled d4
High purity Aprepitant labeled d4 includes a comprehensive Certificate of Analysis and all supporting analytical data
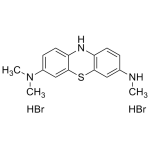
Leuco Azure B Dihydrobromide
High purity Leuco Azure B Dihydrobromide includes a comprehensive Certificate of Analysis and all supporting analytical data
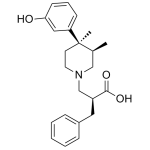
Alvimopan Metabolite
High purity Alvimopan Metabolite includes a comprehensive Certificate of Analysis and all supporting analytical data
N-Nitroso-Atomoxetine
High purity N-Nitroso-Atomoxetine includes a comprehensive Certificate of Analysis and all supporting analytical data
Almorexant labeled d6 Hydrochloride
Almorexant labeled d6 Hydrochloride includes a comprehensive Certificate of Analysis and all supporting analytical data
Apixaban labeled d6
High purity Apixaban labeled d6 includes a comprehensive Certificate of Analysis and all supporting analytical data
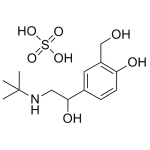
Albuterol
High purity Albuterol includes a comprehensive Certificate of Analysis and all supporting analytical data